出産時低体重児へのビタミンAと早期のBCGワクチンの投与は、死亡率を有意に改善せず。
Vitamin A supplementation and BCG vaccination at birth in low birthweight neonates: two by two factorial randomised controlled trial
BMJ 2010;340:c1101
*全文が読めます。
(メモ)
・two by two factorial randomised controlled trial
・2.5kg未満の低体重児に25 000 IU のビタミンA投与(もしくはプラセボ)、BCGを早期に(もしくは通常期に)投与で、12ヶ月フォロー。第一のアウトカムは、死亡率。
・男女合計はプラセボと有意差がないが、女児には害があるかも知れない
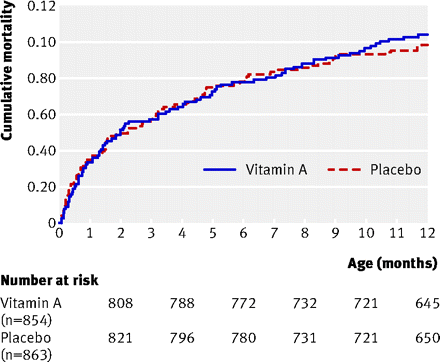
Cumulative mortality during the first year of life for all children
Cumulative mortality during the first year of life for boys
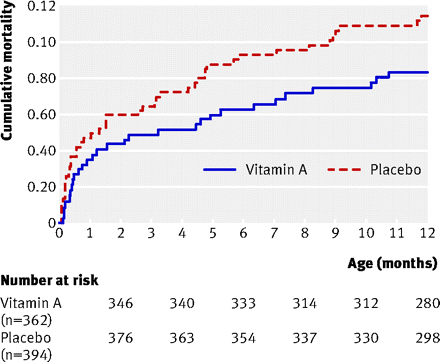
Cumulative mortality during the first year of life for girls
Objective To investigate the effect of vitamin A supplementation and BCG vaccination at birth in low birthweight neonates.
Design Randomised, placebo controlled, two by two factorial trial.
Setting Bissau, Guinea-Bissau.
Participants 1717 low birthweight neonates born at the national hospital.
Intervention Neonates who weighed less than 2.5 kg were randomly assigned to 25 000 IU vitamin A or placebo, as well as to early BCG vaccine or the usual late BCG vaccine, and were followed until age 12 months.
Main outcome measure Mortality, calculated as mortality rate ratios (MRRs), after follow-up to 12 months of age for infants who received vitamin A supplementation compared with those who received placebo.
Results No interaction was observed between vitamin A supplementation and BCG vaccine allocation (P=0.73). Vitamin A supplementation at birth was not significantly associated with mortality: the MRR of vitamin A supplementation compared with placebo, controlled for randomisation to “early BCG” versus “no early BCG” was 1.08 (95% CI 0.79 to 1.47). Stratification by sex revealed a significant interaction between vitamin A supplementation and sex (P=0.046), the MRR of vitamin A supplementation being 0.74 (95% CI 0.45 to 1.22) in boys and 1.42 (95% CI 0.94 to 2.15) in girls. When these data were combined with data from a complementary trial among normal birthweight neonates in Guinea-Bissau, the combined estimate of the effect of neonatal vitamin A supplementation on mortality was 1.08 (95% CI 0.87 to 1.33); 0.80 (95% CI 0.58 to 1.10) in boys and 1.41 (95% CI 1.04 to 1.90) in girls (P=0.01 for interaction between neonatal vitamin A and sex).
Conclusions The combined results of this trial and the complementary trial among normal birthweight neonates have now shown that, overall, it would not be beneficial to implement a neonatal vitamin A supplementation policy in Guinea-Bissau. Worryingly, the trials show that vitamin A supplementation at birth can be harmful in girls. Previous studies and future trials should investigate the possibility that vitamin A supplementation has sex differential effects.
これから)図書館、掃除、ららぼーと、薬害ヤコブの花見、来週からランニング再開(したい)


コメント